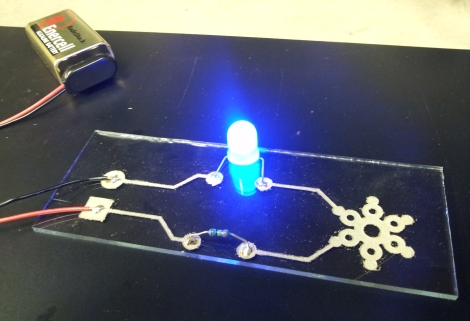
[Jordan] likes the flexibility that conductive inks offer when putting together electronic circuits, but says that they are often too expensive to purchase in decent quantities, and that they usually require substrate-damaging temperatures to cure. After reading a UIUC Materials Research Lab article about making conductive ink that anneals at relatively low temperatures, he decided to give it a shot.
[Jordan] started out by picking up various chemicals and lab supplies online, setting up shop at Pumping Station: One. The process is pretty straightforward, and seems like something just about anyone who took high school chemistry can manage. That said, he does note that some of the chemicals, such as Formic Acid, can be quite painful if mishandled.
After just a few minutes of work and about 12 hours waiting time, [Jordan] had himself a decently-sized vial of conductive ink. He tried it out on a few different substrates with varying results, and in the end found that etched glass made the best circuits. He says that there are plenty of experiments to try, so expect even more helpful info from him in the near future.
[via Pumping Station: One]




Wow, great work Jordan! I can imagine many useful areas. I am happy to see how the innovation comes from amateurs rather than profit based companies. hackerspaces are great places for this.
What’s wrong with profit? Profit creates wealth and jobs. If your employer didn’t make a profit, you wouldn’t have a job, nobody would.
I like it too, and welcome the DIY community to make things as they see fit at a cost they choose to invest. That’s true freedom.
For me, the $12 cost of a premade conductive ink pen makes me buy the pen rather than try this out of convenience…but being a hack addict, I *must* make some of this! Besides, the pen tips never work in the premade pens anyways.
I used to use the $30-a-bottle rear window defrost repair paint for things like this (and this ink could probably be used to repair defrosters as well!). Resistance might not be an issue, as its usually a series circuit with parallel paths in between L and R sides of the glass. Just tape it off on the break point so you paint a nice line over it like this ====== and not this ===O== lol
pretty cool. so the next thing to do is to figure out how to combine this with a MakerBot. 😉
Awesome stuff Jordan (From a fellow PS:One’er)
I wonder if this chemistry could be used to silver glass for the purpose of making mirrors or reflectors.
I nearly fell off my chair then man!
Please don’t say “silver” mirrors!
One of the dangers of “silvering” mirrors is…
Silver Fulminate!!
After spending days/weeks grinding a mirror the last thing you need to see is this black crap appearing through the mirror you are trying to make shiny!
Silver Fulminate, Mercury Fulminate, Lead Azide, Ammonium TriIodide are just too unstable.
Having an 8″ mirror you spent ages grinding, “figuring” and polishing blown into the air or your face is painful on many levels!
Building a vacuum system and a high voltage system to put on an aluminium coating is way less hassle.
Not only that, if you “silver” a mirror, you will have to re-silver it every couple of years.
Climb back in your chair and chill.
Nobody proposed making telescope mirrors using this technique. I merely floated the idea that the ability to lay silver onto glass might have other applications.
“Silvering,” by the way, is a colloquial term for making mirrors “shiny”. It’s not a literal or technical term describing the chemistry used. People have been “silvering” glass mirrors (with a whole host of different materials) for centuries.
As to the risk of explosions, the fact that this guy laid his silver on glass and then repeatedly applied a soldering iron to it without deleterious effect suggests that you might be overreacting.
A fulminate percussion cap, for example, would not tolerate that kind of treatment.
Mercury and azides are not required for mirrors, commercial mirrors are made using a version of the ‘Tollens silver mirror test’ with a dash of tin chloride to make it stick.
http://www.rsc.org/Education/EiC/issues/2007Jan/ExhibitionChemistry.asp
printing silver is just cool.
with 9ohm/cm the resistance seems still high (i use nichrome toaster wire for led drivers and get 0.2ohm/cm)
maybe printing the circuit and then depositing cu on it would help?
I’ll admit that it still is higher than I’d like, but it’s better than a lot of other DIY conductive inks.
Next I’ll be testing out mixing the solution with surfactants and coagulants to see if I can get the silver to spread more uniformly with lower resistance.
*note – the majority of the testing was done at 4am with no sleep, so there’s still a lot of experimenting to be done and progress to be made!
The resistance is a problem, but not an insurmountable one. I’ve been playing with the idea of electroplating over silver ink to solve this very problem, so this fits in well with what I’ve been doing. How is the adhesion of your ink on something like fibreglass? Is pre-etching required to make it work well? Does it stick to plastic?
Badass!
Pity that to make use of the ink, you actually need to laser-engrave a plate of glass.
This isn’t really a solution for anything, is it? It’s just something you can do if you have thousands of dollars worth of equipment.
Actually, you don’t *need* laser etched glass, it’s just what I tested on. The UIUC paper mentions a variety of plastics you can print on as well!
And can those plastics take the heat of a soldering iron?
I agree, especially when the talk was about substrate damaging temperatures. You have to have quite high temperatures to damage glass in any way so you could’ve used any of the other possibilities with it.
@Dax-
This, like many postings, was not advertised as a solution.
@Elias-
Read the article – there were no substrate damaging temperatures.
Give the criticism a rest. This was experiment #1. I think it is great info! If folks go around bashing experiments, the few experimenters will ‘go dark’ until they get it right – thus slowing the rate of innovation. If someone performs an experiment that leads to ANY new knowledge, let’s hear about it. If it does not inspire or appeal to you, keep quiet wrt negative comments. This is not in any way meant to discourage debate.
Is it time to start looking for some of those ancient pen plotters??
You can etch glass chemically: http://makeprojects.com/Project/Label-Etch-a-Glass-Bottle/179/1. Maybe printer toner would be a good resist for this?
The guys tutorial for etching glass with chemicals says:
Glass etchants are toxic and should be handled with care. Wear gloves and goggles and follow the label directions closely.
yet in all of his pictures, he’s not wearing any type of protection…
:facepalm: Really, Dax? A guy comes up with a substance that, even in its current, early form, can be used to form printed conductors, and all you have to say is “This isn’t really a solution for anything, is it?” To misquote Benjamin Franklin, “What is the use of an infant, trollboy?”
Hmmm… Extruded conductor + Extruded insulator + pick-n-place = 3D, structured circuitry. You project case and/or supports *contain* your circuitry.
While I agree with you wholeheartedly, this is decidedly a 2D conductor. It condenses into a film and, I suspect, would not be suitable for producing a 3D structures, or vertical columns, if you will. I hold out hope for a solution on that score, but there doesn’t seem to be a viable one yet.
The best solution I’ve been able to come up with for 3D conductive structures is a composite of copper powder and weakly conductive plastic (3M makes a conductive urethane which is compatible with most printed plastics: it has a 200C melting point). The problem with this is that the composite has to be manufactured–that is that copper powder has to be produced and blended with the liquid conductive urethane–and that I suspect the copper powder suspension would jam most extruders.
Comment is not hack related – In before political rhetoric starts getting spewed back and forth. *rolleyes*
I wonder if I can use this as a hole wall activator so I can then do plated through vias? I have been looking for something like this and the only one I could find was very expensive. This looks promising.
That’s a darn fine idea there. 🙂
Heck, it’d be especially great if it adheres to holes that aren’t cut particularly smoothly, or if it could be used right on the perimeter of a board.
Could it be ‘hybridized’ with adhesives, or an epoxy? I’m thinking if its viscosity could be increased, there would be a few more niches this conductive ink could fill.
Anyhoo, very nice work AND documentation, Jordan. Best of luck on continuing with this line of experimentation; please be sure to keep us updated on progress. I’m definitely interested in learning more.
That is a damn fine idea! I’ll be sure to try it! I think I’ve got some double-sided copper clad somewhere…
Also: as I mentioned above, I’m planning on mixing this with some coagulants and surfactants to see if I can change the viscosity and resistance of the solution.
Most likely, I’ll probably try to have a beer or two with the researchers themselves if I can get in touch with them! They’re not *too* far away from where I’m at, and I’m sure they’d have lots of tips and tricks!
The procedure for another ink I read about was to squeegee the ink over the holes then use a vacuum to suck out excess from the holes on the other side, then cure for a while. Then electroplate. In theory 🙂
So is anyone going to try putting this into an inkjet cartridge? A cart full of this and a piece of glass roughed up with glass etchant put in your cd printer and your all set.
How long does the mixed “ink” keep? Data on similar chemical mixes (Tollens, Fehling) say that they should be prepared immediately before use, and kept no longer than 24hours, but this is a somewhat different mix.
(part of the caution is that explosion risk. It
s not the deposited silver, nor fulminate, but some nasty silver/ammonia/nitrogen complexes that can form in the solution (don’t let your leftovers dry up!) (I don’t know whether you’d get the same issues starting with acetate…)
Have you done any experiments adding thickeners to the final solution?
after reading the paper and you experiments i had a couple of thoughts:
if someone wants to replicate this on a budget i think you could cut back on some of the lab equipment you bought.
since your not using the ink in a printer (which could clog if bigger particles are present) i think the microfilters are a bit overkill. using a needle to aspirate the clear fluid after its setteled is probably enought.
also i was wondering how you came up with the quantities did you calulate them based on the molecular weight and purity of you chemicals?
also from what i read in the paper there is still 46% silver acetate after the drying period. i wonder if you could use (thats going to sound crazy but anyway) a microwave or an induction heating to speed up the drying process. my initial thought was that the em waves will selectively heat up the silver nanocristall which would accelerate the evaporation process and if lucky maybe even thermolyse some of the silver acetate.
I also wonder if it would be possible to cure a formulation with UV light.
also,
since doesn’t affect ketones, maybe paint onto Tyvek or even teflon tape.
microwave after on teflon, might roll up to protect lines too…
I am not a polymer chemist, but I bet if you headed to your local hardware store and picked up a metallic spray ( or easier – liquid metallic paint ) you could attain the same result.
Cheers
one thing where a higher resistance wouldn’t be a problem: capacitive sensors.
making your own custom sensor pads, buttons. maybe even on flexible material. or a very rough touchscreen that would add a couple virtual buttons to a cellphone lcd.
another idea:
print your antenna design